
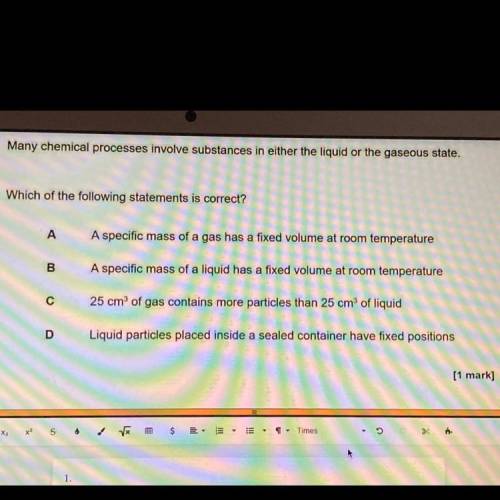
Many chemical processes involve substances in either the liquid or the gaseous state.
Which of the following statements is correct?
A. A specific mass of a gas has a fixed volume at room temperature
B. A specific mass of a liquid has a fixed volume at room temperature
C. 25 cm of gas contains more particles than 25 cm of liquid
D. Liquid particles placed inside a sealed container have fixed positions
[1 mark]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3


Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1

You know the right answer?
Many chemical processes involve substances in either the liquid or the gaseous state.
Which of the...
Questions

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Geography, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

English, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Biology, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01



