
Chemistry, 05.05.2021 08:30 2020seogang
You have a container of 5.0 L with a molarity of 0.750 M. What would be the new volume if you increased the concentration of the solution to 9.71 M?
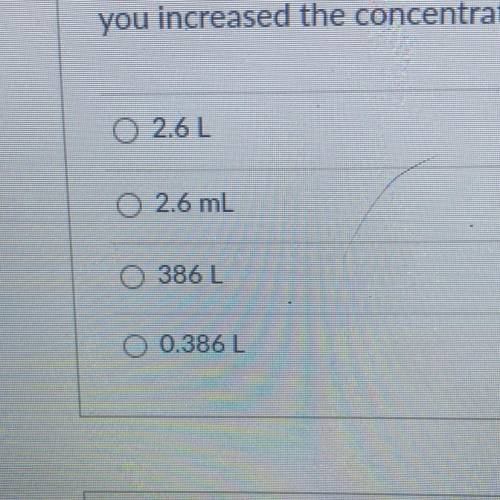

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
You have a container of 5.0 L with a molarity of 0.750 M. What would be the new volume if
you incr...
Questions

Mathematics, 07.09.2021 14:00


Biology, 07.09.2021 14:00

Geography, 07.09.2021 14:00


English, 07.09.2021 14:00

Mathematics, 07.09.2021 14:00

English, 07.09.2021 14:00

Biology, 07.09.2021 14:00

English, 07.09.2021 14:00


Mathematics, 07.09.2021 14:00


English, 07.09.2021 14:00


Mathematics, 07.09.2021 14:00

Computers and Technology, 07.09.2021 14:00

Computers and Technology, 07.09.2021 14:00


Mathematics, 07.09.2021 14:00



