Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
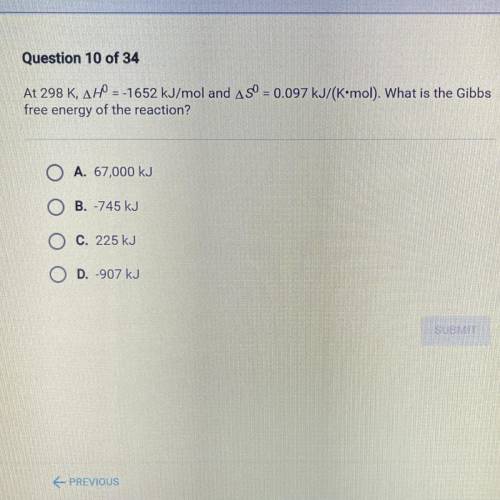
At 298 K, AH = -1652 kJ/mol and ASO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the react...
Questions

Mathematics, 20.11.2021 09:20


Mathematics, 20.11.2021 09:20

Mathematics, 20.11.2021 09:20

Computers and Technology, 20.11.2021 09:20

Social Studies, 20.11.2021 09:20

Mathematics, 20.11.2021 09:20


Mathematics, 20.11.2021 09:20

Mathematics, 20.11.2021 09:20

Geography, 20.11.2021 09:20




History, 20.11.2021 09:20




Mathematics, 20.11.2021 09:20

Mathematics, 20.11.2021 09:20