
Chemistry, 05.05.2021 02:10 peachijmin
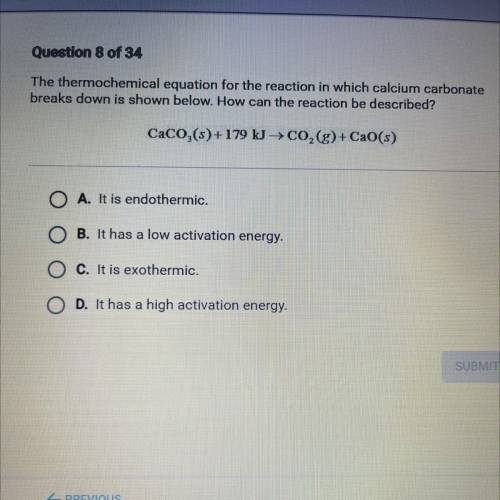
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below. How can the reaction be described?
CaCO,(s)+179 kg CO,(g)+ CaO(s)
A. It is endothermic.
B. It has a low activation energy.
C. It is exothermic.
D. It has a high activation energy.
NO LINKS

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below...
Questions


Mathematics, 27.07.2020 19:01

Biology, 27.07.2020 19:01






English, 27.07.2020 19:01

English, 27.07.2020 19:01

Mathematics, 27.07.2020 19:01



Mathematics, 27.07.2020 19:01


History, 27.07.2020 19:01




Mathematics, 27.07.2020 19:01



