Chemistry, 04.05.2021 23:00 faithlopez209
PLEASE HELP, WILL GIVE BRAINLIEST
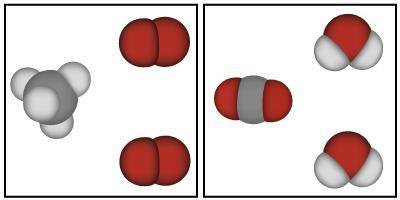
The image below shows models that represent the reactants and products of a chemical reaction.
Based on the image below, which of the following is true?
A. Mass could have either been gained or lost during this chemical reaction because atoms always change mass when they react.
B. Mass was lost in this chemical reaction because the atoms in the reactants are smaller than the atoms in the products.
C. Mass was conserved in this chemical reaction because the same atoms are present in both the products and the reactants.
D. Mass was gained in this chemical reaction because there are more atoms in the products than there were in the reactants.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
PLEASE HELP, WILL GIVE BRAINLIEST
The image below shows models that represent the reactants and pr...
Questions













Engineering, 06.11.2020 16:20


Computers and Technology, 06.11.2020 16:20

Mathematics, 06.11.2020 16:20



Mathematics, 06.11.2020 16:20