
Chemistry, 03.05.2021 19:00 kcutler8603
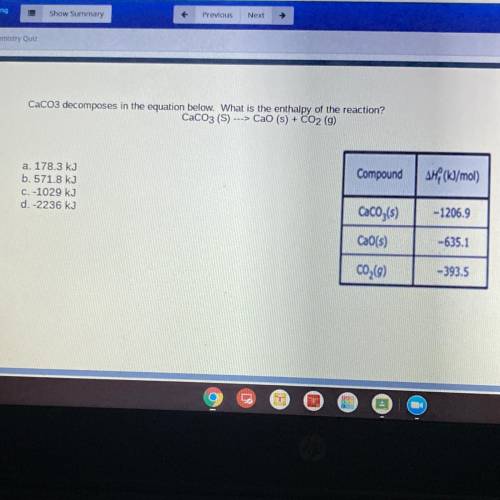
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Cao (s) + CO2 (g)
Compound
AH (kJ/mol)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d.-2236 kJ
CaCO3(s)
-1206.9
CaO(s)
-635.1
C02(9)
-393.5

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions


Social Studies, 16.07.2019 22:00



English, 16.07.2019 22:00


History, 16.07.2019 22:00

Health, 16.07.2019 22:00

Biology, 16.07.2019 22:00







English, 16.07.2019 22:00

Chemistry, 16.07.2019 22:00

Social Studies, 16.07.2019 22:00

Chemistry, 16.07.2019 22:00



