
Chemistry, 03.05.2021 18:50 datgamer13
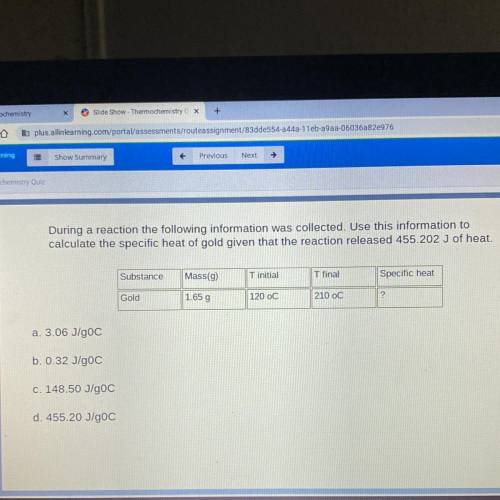
During a reaction the following information was collected. Use this information to
calculate the specific heat of gold given that the reaction released 455.202 J of heat.
Substance
Mass(9)
T initial
T final
Specific heat
Gold
1.65 g
120 oC
210 OC
2
a. 3.06 J/gOC
b. 0.32 J/goC
c. 148.50 J/goC
d. 455.20 J/goC

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
During a reaction the following information was collected. Use this information to
calculate the s...
Questions


English, 22.11.2020 22:50







Mathematics, 22.11.2020 23:00





Mathematics, 22.11.2020 23:00

Geography, 22.11.2020 23:00

English, 22.11.2020 23:00


Geography, 22.11.2020 23:00




