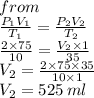Chemistry, 03.05.2021 18:30 katherine3084
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases to
35.0°C and the pressure decreases to 1.00 atm, what is the new volume?
a. 1630 mL
b. 138 ml
c. 163 mL
d. 0.0017mL

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases...
Questions

English, 25.06.2020 02:01

Mathematics, 25.06.2020 02:01



Mathematics, 25.06.2020 02:01

Mathematics, 25.06.2020 02:01




Mathematics, 25.06.2020 02:01







Geography, 25.06.2020 02:01


History, 25.06.2020 02:01

Mathematics, 25.06.2020 02:01