
Chemistry, 03.05.2021 14:00 juliaduenkelsbu
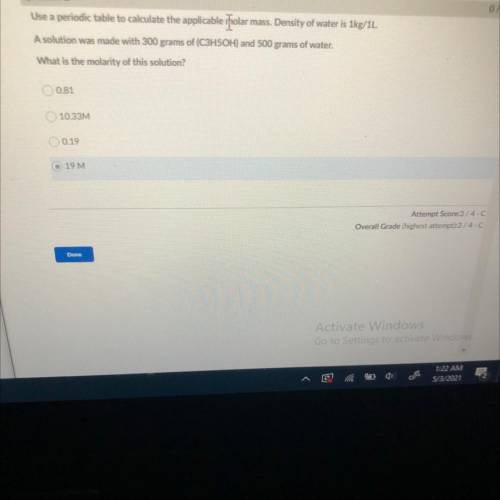
Use a periodic table to calculate the applicable rolar mass. Density of water is 1kg/1L.
A solution was made with 300 grams of (C3H5OH) and 500 grams of water.
What is the molarity of this solution?
0.81
10.33M
0.19
19 M
D or 19 M is not correct.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
Use a periodic table to calculate the applicable rolar mass. Density of water is 1kg/1L.
A solutio...
Questions

Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20




Health, 19.04.2021 17:20




Mathematics, 19.04.2021 17:20


Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20


English, 19.04.2021 17:20


Mathematics, 19.04.2021 17:20

Mathematics, 19.04.2021 17:20

Health, 19.04.2021 17:20




