What is the mass of each of the following?
(a) 5.34 x 10-2 mol CuCl2
(b) 1.50 X 102 mol Ca(O...

Chemistry, 02.05.2021 18:40 kutemigos9211
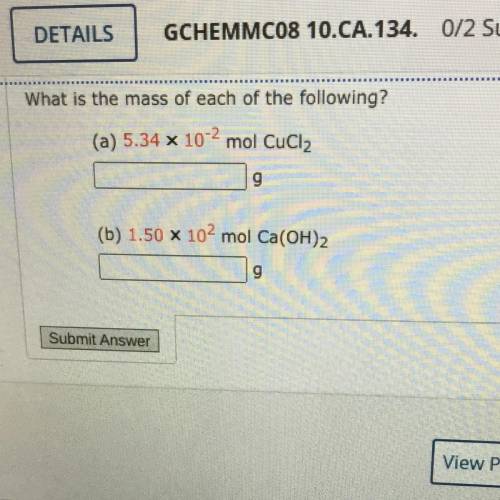
What is the mass of each of the following?
(a) 5.34 x 10-2 mol CuCl2
(b) 1.50 X 102 mol Ca(OH)2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Questions

English, 31.07.2019 23:00

English, 31.07.2019 23:00


Computers and Technology, 31.07.2019 23:00

History, 31.07.2019 23:00





Physics, 31.07.2019 23:00


Physics, 31.07.2019 23:00

History, 31.07.2019 23:00



Chemistry, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00


Mathematics, 31.07.2019 23:00



