
Chemistry, 02.05.2021 01:00 lilzaya510
A model of tin, an element with the atomic number 50, is shown here. The valence electrons are modeled here in this image. Which statements are supported by the information in the model? Select ALL That apply.
A) Tin needs four more electrons to complete its outer shell.
B) Tin has no neutrons in the nucleus, as is shown in the model.
C) Tin is highly reactive because it only has four valence electrons.
D) Tin is negatively charged because it more electrons than protons.
E) Tin has a low reactivity because it has full inner shells of electrons.
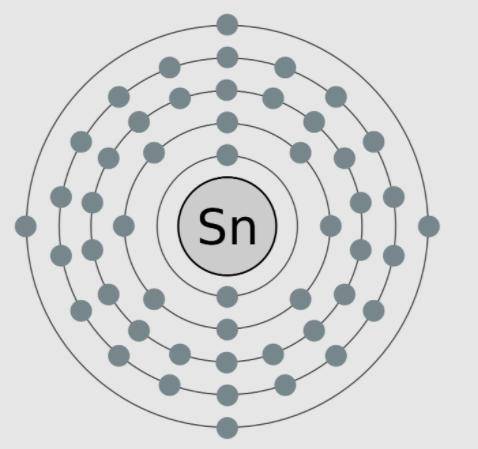

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3
You know the right answer?
A model of tin, an element with the atomic number 50, is shown here. The valence electrons are model...
Questions

Social Studies, 03.02.2021 08:20



History, 03.02.2021 08:20

Mathematics, 03.02.2021 08:20


Chemistry, 03.02.2021 08:20


Mathematics, 03.02.2021 08:20

History, 03.02.2021 08:20







History, 03.02.2021 08:20

Mathematics, 03.02.2021 08:20


Social Studies, 03.02.2021 08:20



