
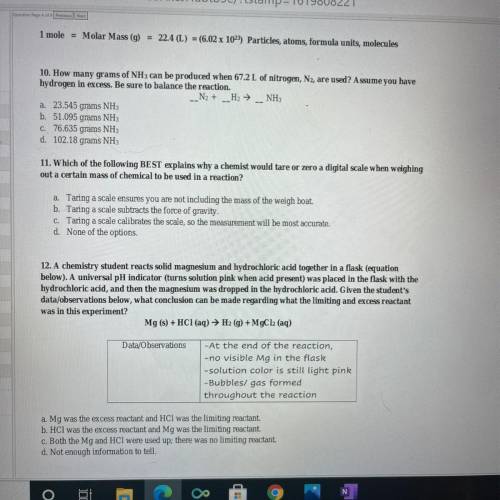
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2 hydrogen in excess. Be sure to balance the reaction. 12. A chemistry student reacts solid magnesium and hydrochloric acid together in a flask (equation below). A universal pH indicator (turns solution pink when acid present) was placed in the flask with the hydrochloric acid, and then the magnesium was dropped in the hydrochloric acid. Given the student's data/ observations below, what conclusion can be made regarding what the limiting and excess reactant was in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2...
Questions


Social Studies, 03.11.2019 18:31

Mathematics, 03.11.2019 18:31



Mathematics, 03.11.2019 18:31

World Languages, 03.11.2019 18:31




Mathematics, 03.11.2019 18:31


English, 03.11.2019 18:31

History, 03.11.2019 18:31


Social Studies, 03.11.2019 18:31

Biology, 03.11.2019 18:31


English, 03.11.2019 18:31



