
Chemistry, 30.04.2021 19:40 james22000
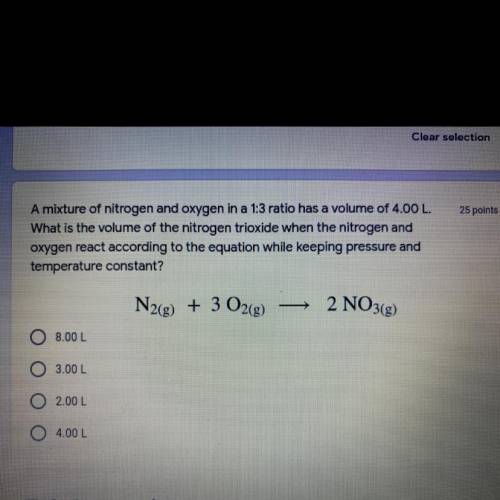
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the nitrogen trioxide when the nitrogen and
oxygen react according to the equation while keeping pressure and temperature constant?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the...
Questions

Mathematics, 24.12.2020 01:00



Arts, 24.12.2020 01:00


Mathematics, 24.12.2020 01:00

Mathematics, 24.12.2020 01:00


French, 24.12.2020 01:00







Mathematics, 24.12.2020 01:00



Mathematics, 24.12.2020 01:00



