PLEASE HELP ME classify this reaction
your options are
1. synthesis
2. decomposition<...

Chemistry, 30.04.2021 16:40 kingsavage2002
PLEASE HELP ME classify this reaction
your options are
1. synthesis
2. decomposition
3. combustion
4. single replacement
5. double replacement
explain your answer for brainliest
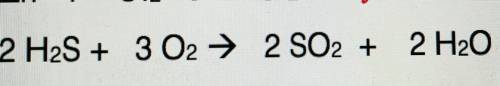

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Questions

English, 17.04.2020 12:50

Mathematics, 17.04.2020 12:50

History, 17.04.2020 12:51



History, 17.04.2020 12:51

Biology, 17.04.2020 12:51

Mathematics, 17.04.2020 12:51

Mathematics, 17.04.2020 12:51



Mathematics, 17.04.2020 12:51

Mathematics, 17.04.2020 12:51


English, 17.04.2020 12:51

Mathematics, 17.04.2020 12:51


Mathematics, 17.04.2020 12:51

Mathematics, 17.04.2020 12:52



