
Chemistry, 30.04.2021 16:30 woodfordmaliky
When 14.8 grams of an ionic substance ( cation + anion have the same charge ) is dissolved in 125.0 g of ethanol , the solution begins to boil at 81.2C Calculate the molar mass of this solute. (i need work aswell)
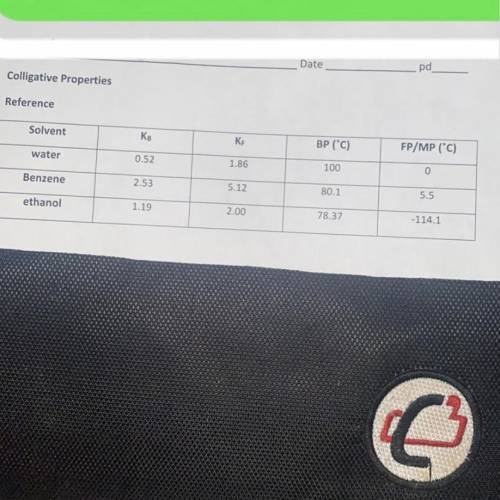

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

You know the right answer?
When 14.8 grams of an ionic substance ( cation + anion have the same charge ) is dissolved in 125.0...
Questions

English, 01.10.2019 01:00

History, 01.10.2019 01:00

Chemistry, 01.10.2019 01:00


Mathematics, 01.10.2019 01:00

Mathematics, 01.10.2019 01:00










English, 01.10.2019 01:00

History, 01.10.2019 01:00


Mathematics, 01.10.2019 01:00



