
Chemistry, 30.04.2021 09:10 LarryJoeseph
How many liters of water vapor is produced when 33.4 moles of gaseous ammonia is reacted in the following reaction? Hint: Convert to moles first
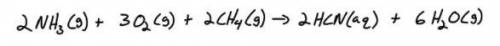

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
How many liters of water vapor is produced when 33.4 moles of gaseous ammonia is reacted in the foll...
Questions

Biology, 15.12.2019 09:31

Computers and Technology, 15.12.2019 09:31



History, 15.12.2019 09:31

English, 15.12.2019 09:31

Mathematics, 15.12.2019 09:31

Chemistry, 15.12.2019 09:31


Business, 15.12.2019 09:31



Physics, 15.12.2019 09:31

Mathematics, 15.12.2019 09:31





Biology, 15.12.2019 09:31



