
Chemistry, 30.04.2021 05:00 randlemccray6338
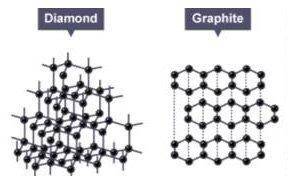
Consider the two models seen here. These models represent two different minerals. Each is composed of a lattice of carbon atoms. How do these crystals differ?
A) The two have different chemical formulas. Eliminate
B) The diamond model represents a crystal while the graphite represents a molecule.
C) Both are crystals, but the organized arrangement of the carbon atoms in each differs.
D) The graphite crystal is not pure: that is, it contains two elements while the diamond crystal is composed of carbon atoms only.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Consider the two models seen here. These models represent two different minerals. Each is composed o...
Questions



Mathematics, 25.05.2021 02:50

Mathematics, 25.05.2021 02:50




Chemistry, 25.05.2021 02:50






Mathematics, 25.05.2021 02:50



Mathematics, 25.05.2021 02:50



Chemistry, 25.05.2021 02:50




