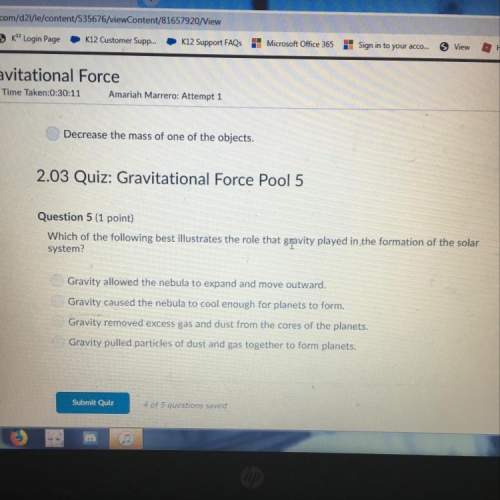
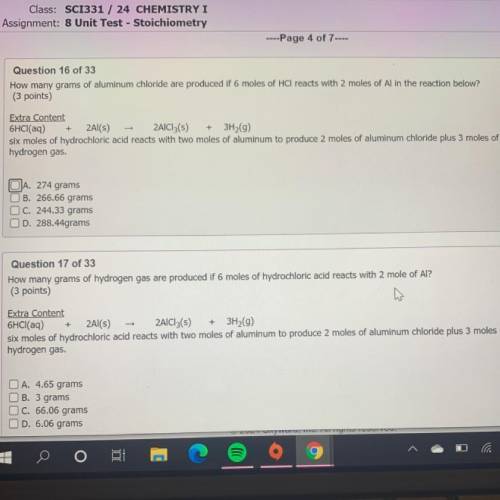
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the reaction below?
(3 points)
Extra Content
6HCl(aq) + 2Al(s) - 2AlCl3(s) + 3H2(9)
six moles of hydrochloric acid reacts with two moles of aluminum to produce 2 moles of aluminum chloride plus 3 moles of
hydrogen gas.
JA. 274 grams
B. 266.66 grams
C. 244.33 grams
D. 288.44grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the...
Questions

Computers and Technology, 17.06.2020 23:57





History, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57



Mathematics, 17.06.2020 23:57


Mathematics, 17.06.2020 23:57


Biology, 17.06.2020 23:57

Geography, 17.06.2020 23:57




Mathematics, 17.06.2020 23:57