
Chemistry, 29.04.2021 22:20 amortegaa805
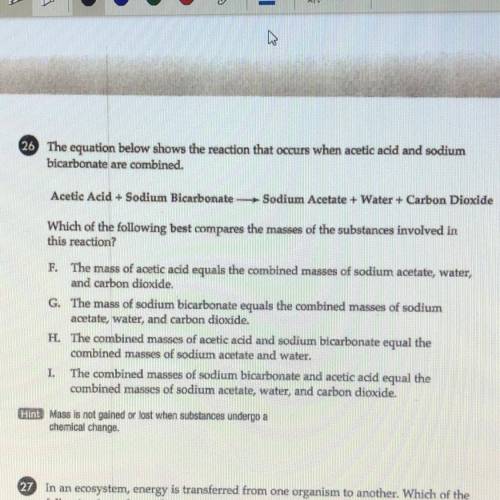
WILL MARK BRAINLIEST PLZ HURRY. 26 The equation below shows the reaction that occurs when acetic acid and sodium
bicarbonate are combined.
Acetic Acid + Sodium Bicarbonate Sodium Acetate + Water + Carbon Dioxide
Which of the following best compares the masses of the substances involved in
this reaction?
F. The mass of acetic acid equals the combined masses of sodium acetate, water,
and carbon dioxide.
G. The mass of sodium bicarbonate equals the combined masses of sodium
acetate, water, and carbon dioxide.
H. The combined masses of acetic acid and sodium bicarbonate equal the
combined masses of sodium acetate and water.
I. The combined masses of sodium bicarbonate and acetic acid equal the
combined masses of sodium acetate, water, and carbon dioxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3


Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
WILL MARK BRAINLIEST PLZ HURRY. 26 The equation below shows the reaction that occurs when acetic aci...
Questions

History, 30.08.2019 00:30

History, 30.08.2019 00:30

Biology, 30.08.2019 00:30

Computers and Technology, 30.08.2019 00:30

English, 30.08.2019 00:30



Mathematics, 30.08.2019 00:30






Mathematics, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30

History, 30.08.2019 00:30

English, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30

History, 30.08.2019 00:30



