
Chemistry, 29.04.2021 20:40 dontcareanyonemo
24g of methane were burned in an excess of air. What mass of water would be produced in the reaction assuming complete combustion?Use the information below to answer the question.
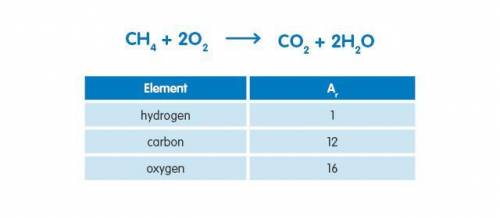

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
24g of methane were burned in an excess of air. What mass of water would be produced in the reaction...
Questions

History, 07.04.2021 19:50



English, 07.04.2021 19:50

Mathematics, 07.04.2021 19:50


Mathematics, 07.04.2021 19:50


Mathematics, 07.04.2021 19:50

Mathematics, 07.04.2021 19:50








Mathematics, 07.04.2021 19:50




