
Chemistry, 29.04.2021 06:30 steven122048i
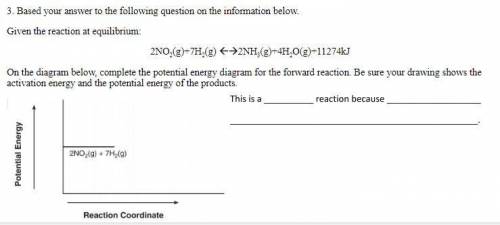
Based your answer to the following question on the information below.
Given the reaction at equilibrium:
2NO2(g)+7H2(g) 2NH3(g)+4H2O(g)+11274kJ
On the diagram below, complete the potential energy diagram for the forward reaction. Be sure your drawing shows the activation energy and the potential energy of the products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
Based your answer to the following question on the information below.
Given the reaction at equili...
Questions

English, 18.03.2020 03:46


Mathematics, 18.03.2020 03:46


Mathematics, 18.03.2020 03:46

Physics, 18.03.2020 03:46

Computers and Technology, 18.03.2020 03:46

Mathematics, 18.03.2020 03:46

Mathematics, 18.03.2020 03:46


English, 18.03.2020 03:46

Physics, 18.03.2020 03:46



Mathematics, 18.03.2020 03:46


Mathematics, 18.03.2020 03:46

History, 18.03.2020 03:46

Mathematics, 18.03.2020 03:46



