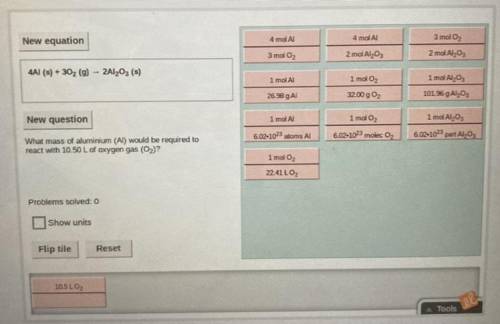
Equation: 4Al (s) + 3O2 (g) —> 2Al2O3 (s)
(Solve using stoichiometry)
...

Chemistry, 29.04.2021 01:00 student0724
Equation: 4Al (s) + 3O2 (g) —> 2Al2O3 (s)
(Solve using stoichiometry)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

You know the right answer?
Questions

Mathematics, 16.12.2020 23:50






History, 16.12.2020 23:50

Mathematics, 16.12.2020 23:50





Geography, 16.12.2020 23:50


Mathematics, 16.12.2020 23:50







