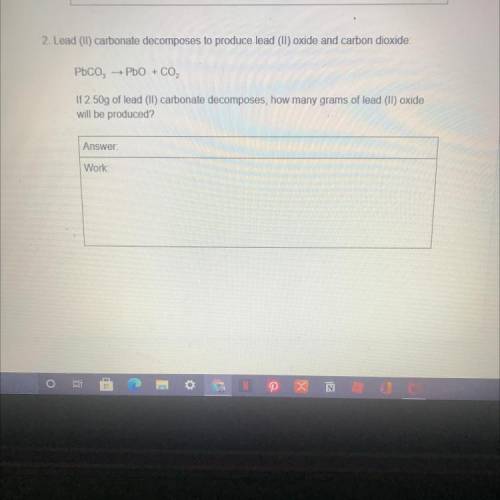
2. Lead (II) carbonate decomposes to produce lead (II) oxide and carbon dioxide:
Pbco,
→ PbO...

Chemistry, 28.04.2021 21:20 yulaarmstrong
2. Lead (II) carbonate decomposes to produce lead (II) oxide and carbon dioxide:
Pbco,
→ PbO + CO2
If 2.50g of lead (II) carbonate decomposes, how many grams of lead (II) oxide
will be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1
You know the right answer?
Questions


Biology, 05.07.2021 14:40

Computers and Technology, 05.07.2021 14:40




Social Studies, 05.07.2021 14:50

Mathematics, 05.07.2021 14:50

Biology, 05.07.2021 14:50


Biology, 05.07.2021 14:50

English, 05.07.2021 14:50

Mathematics, 05.07.2021 14:50

Business, 05.07.2021 14:50




Mathematics, 05.07.2021 14:50


Mathematics, 05.07.2021 15:00



