
Chemistry, 28.04.2021 19:50 leandrogarin37p2g5ds
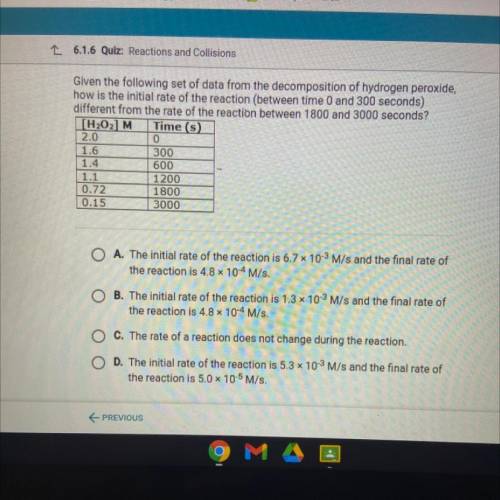
Given the following set of data from the decomposition of hydrogen peroxide,
how is the initial rate of the reaction (between time 0 and 300 seconds)
different from the rate of the reaction between 1800 and 3000 seconds?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Given the following set of data from the decomposition of hydrogen peroxide,
how is the initial ra...
Questions

Mathematics, 05.11.2019 13:31

English, 05.11.2019 13:31




English, 05.11.2019 13:31


English, 05.11.2019 13:31

Business, 05.11.2019 13:31

Social Studies, 05.11.2019 13:31

Mathematics, 05.11.2019 13:31

Mathematics, 05.11.2019 13:31

Health, 05.11.2019 13:31

Social Studies, 05.11.2019 13:31


Health, 05.11.2019 13:31

History, 05.11.2019 13:31

Biology, 05.11.2019 13:31

Social Studies, 05.11.2019 13:31

History, 05.11.2019 13:31



