
Chemistry, 28.04.2021 19:50 loganharper992
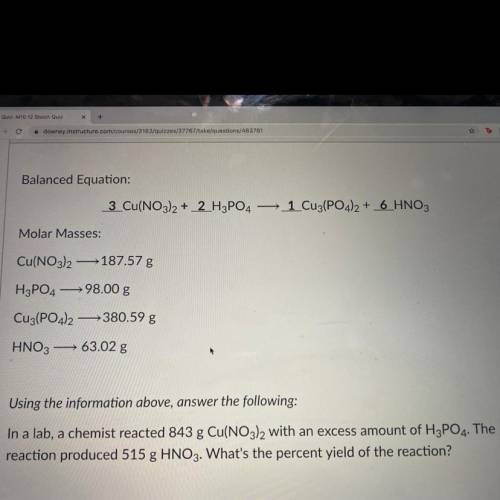
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The reaction produced 515 g HNO3. What's the percent yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The
reaction produced 5...
Questions





English, 08.09.2020 14:01






English, 08.09.2020 14:01











