
Chemistry, 28.04.2021 19:30 rwlockwood1
Please
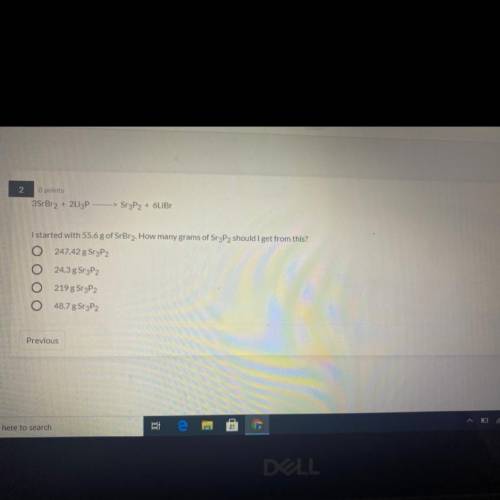
3SrBr2 + 2Li3P —>Sr3P2 + 6LiBr
I started with 55.6 g of SrBr2. How many grams of Sr3P2 should I get from this?
A) 247.42 g SrP2
B) 24.3 g Sr3P2
C) 219 g Sr3P2
D) 48.7 g Sr3P2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Please
3SrBr2 + 2Li3P —>Sr3P2 + 6LiBr
I started with 55.6 g of SrBr2. How many gram...
I started with 55.6 g of SrBr2. How many gram...
Questions

Biology, 06.12.2019 21:31

Mathematics, 06.12.2019 21:31

Geography, 06.12.2019 21:31



Mathematics, 06.12.2019 21:31

Mathematics, 06.12.2019 21:31

History, 06.12.2019 21:31

English, 06.12.2019 21:31

Mathematics, 06.12.2019 21:31




History, 06.12.2019 21:31

English, 06.12.2019 21:31

Social Studies, 06.12.2019 21:31



Mathematics, 06.12.2019 21:31



