Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
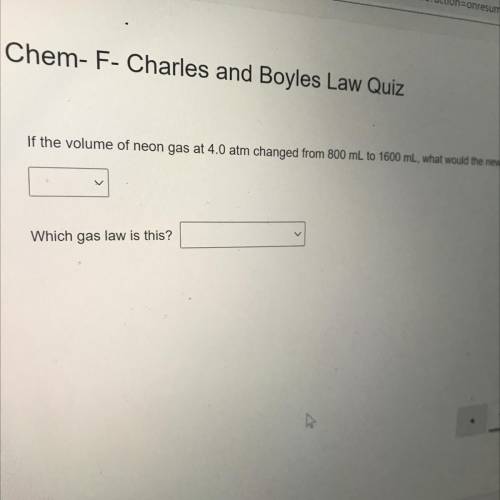
If the volume of neon gas at 4.0 atm changed from 800 mL to 1600 mL, what would the new pressure be?...
Questions


History, 10.03.2021 19:30





Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30


Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30


Mathematics, 10.03.2021 19:30



Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30