Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 13:00
How does the kinetic energy of a substance's particle in the solid phase compare to their kinetic enegy in the liquid phase?
Answers: 1

Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1
You know the right answer?
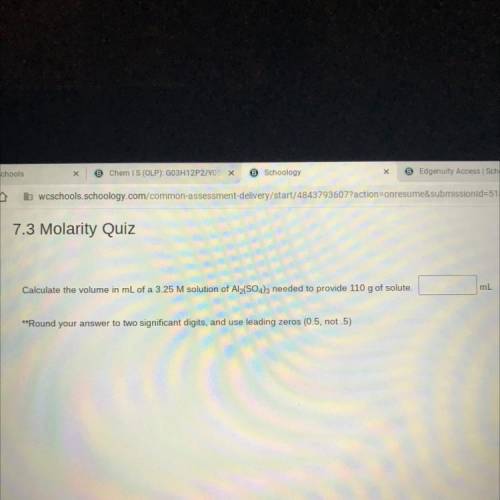
Calculate the volume in mL of a 3.25 M solution of Al2(SO4)3 needed to provide 110 g of solute.
Questions





Medicine, 09.07.2019 21:10




Computers and Technology, 09.07.2019 21:10



Medicine, 09.07.2019 21:10







History, 09.07.2019 21:20