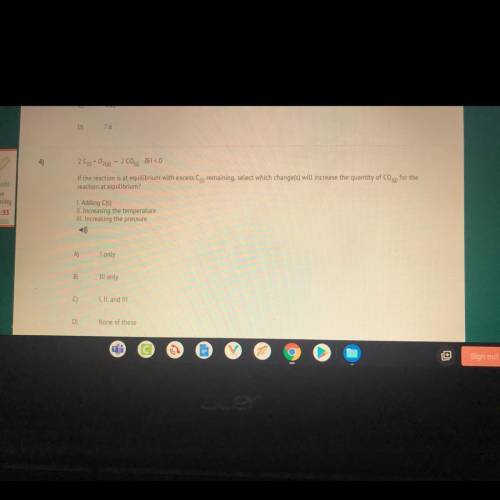
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining,...

Chemistry, 28.04.2021 02:50 mahagonylabeyta
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining, select which change(s) will increase the quantity of Coq) for the
reaction at equilibrium?
I. Adding C(s)
II. Increasing the temperature
III. Increasing the pressure

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

You know the right answer?
Questions

History, 22.09.2021 02:30


English, 22.09.2021 02:30

Mathematics, 22.09.2021 02:30

Mathematics, 22.09.2021 02:30

Computers and Technology, 22.09.2021 02:30








Spanish, 22.09.2021 02:30

Chemistry, 22.09.2021 02:30

Mathematics, 22.09.2021 02:30



Health, 22.09.2021 02:30

Business, 22.09.2021 02:30



