What is the equilibrium constant for the chemical equation:
Mg3P2 (s) ++ 3Mg+2 (aq) + 2P-3
(...

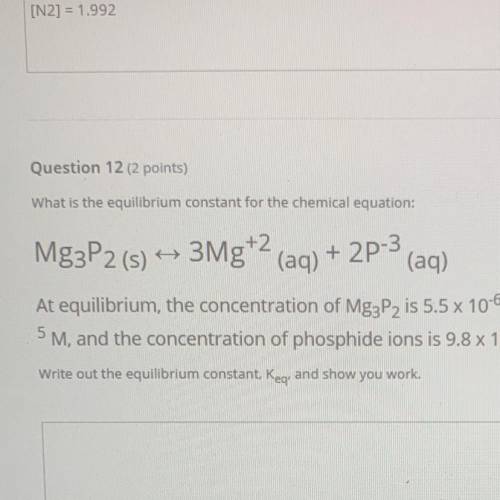
What is the equilibrium constant for the chemical equation:
Mg3P2 (s) ++ 3Mg+2 (aq) + 2P-3
(aq)
At equilibrium, the concentration of Mg3P2 is 5.5 x 10-6 M, the concentration of magnesium ions is 7.2 x 10
5 M, and the concentration of phosphide ions is 9.8 x 10-8 M.
Write out the equilibrium constant, Keg. and show you work.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Questions



English, 03.11.2020 21:20




Health, 03.11.2020 21:20

Mathematics, 03.11.2020 21:20

Mathematics, 03.11.2020 21:20


Mathematics, 03.11.2020 21:20


Biology, 03.11.2020 21:20

Mathematics, 03.11.2020 21:20

Mathematics, 03.11.2020 21:20

Biology, 03.11.2020 21:20


English, 03.11.2020 21:20




