Using the following equation:
Pb(SO4)2 + 4LiNO3 Pb(NO3)4 + 2Li2SO4
How many grams of l...

Chemistry, 27.04.2021 18:20 LilLappyLOL
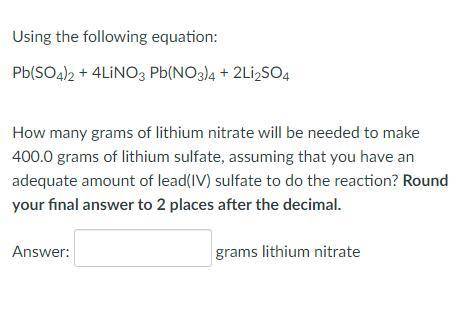
Using the following equation:
Pb(SO4)2 + 4LiNO3 Pb(NO3)4 + 2Li2SO4
How many grams of lithium nitrate will be needed to make 400.0 grams of lithium sulfate, assuming that you have an adequate amount of lead(IV) sulfate to do the reaction? Round your final answer to 2 places after the decimal.
_ grams lithium nitrate

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
Questions

Social Studies, 13.11.2020 20:30

English, 13.11.2020 20:30

Geography, 13.11.2020 20:30

History, 13.11.2020 20:30

Mathematics, 13.11.2020 20:30



Biology, 13.11.2020 20:30







Health, 13.11.2020 20:30




Engineering, 13.11.2020 20:30



