Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
How many grams of sod...

Chemistry, 27.04.2021 18:20 Dashavu4626
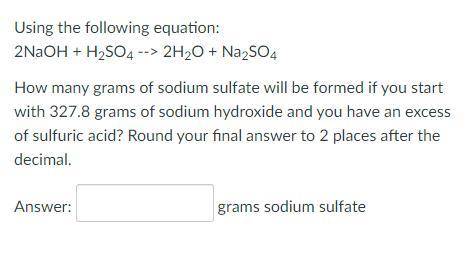
Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
How many grams of sodium sulfate will be formed if you start with 327.8 grams of sodium hydroxide and you have an excess of sulfuric acid? Round your final answer to 2 places after the decimal.
_ grams sodium sulfate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Questions











Mathematics, 29.03.2021 20:40





Physics, 29.03.2021 20:40

History, 29.03.2021 20:40

Mathematics, 29.03.2021 20:40

English, 29.03.2021 20:40

Mathematics, 29.03.2021 20:40



