
Chemistry, 26.04.2021 21:10 tamikagoss22
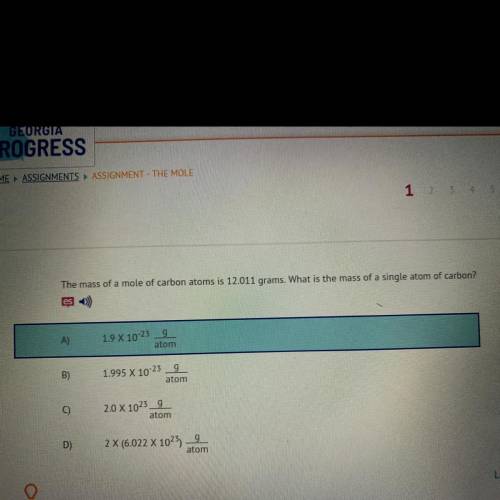
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A)9
1.9 X 10-23
atom
B)
g
1.995 X 10-23
atom
C)
2.0 X 1023_9
atom
D)
2 X (6.022 X 1025)
9
atom
Law of Conserv
Y

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A...
Questions

Social Studies, 12.07.2019 21:00


Biology, 12.07.2019 21:00





English, 12.07.2019 21:00

Mathematics, 12.07.2019 21:00


Advanced Placement (AP), 12.07.2019 21:00



Social Studies, 12.07.2019 21:00


Chemistry, 12.07.2019 21:00

Biology, 12.07.2019 21:00

Biology, 12.07.2019 21:00

Social Studies, 12.07.2019 21:00

History, 12.07.2019 21:00



