Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
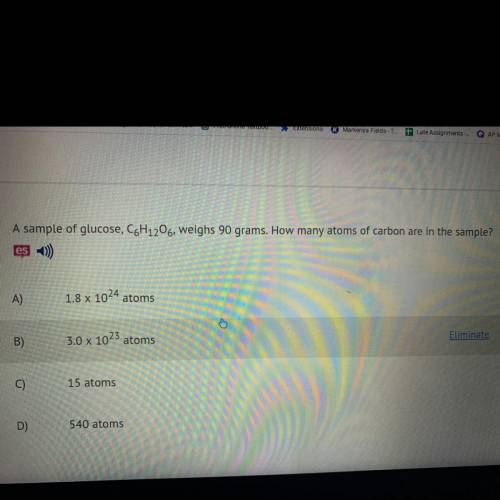
A sample of glucose, C6H12O6, weighs 90 grams. How many atoms of carbon are in the sample?
A)
Questions

Chemistry, 12.01.2021 22:30

Social Studies, 12.01.2021 22:30


SAT, 12.01.2021 22:30

Chemistry, 12.01.2021 22:30


History, 12.01.2021 22:30

History, 12.01.2021 22:30

Health, 12.01.2021 22:30


Spanish, 12.01.2021 22:30

Mathematics, 12.01.2021 22:30

Social Studies, 12.01.2021 22:30



Mathematics, 12.01.2021 22:30


Mathematics, 12.01.2021 22:30