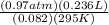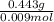Chemistry, 26.04.2021 09:10 kmcgregor4155
An experiment shows that a 236 mL gas sample has a mass of 0.443 g at a pressure of 740 mmHg and a temperature of 22 ∘C. What is the molar mass of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
An experiment shows that a 236 mL gas sample has a mass of 0.443 g at a pressure of 740 mmHg and a t...
Questions

Chemistry, 14.02.2021 09:10

Spanish, 14.02.2021 09:10



Physics, 14.02.2021 09:10


English, 14.02.2021 09:10

History, 14.02.2021 09:10

Mathematics, 14.02.2021 09:20



English, 14.02.2021 09:20


Mathematics, 14.02.2021 09:20

Mathematics, 14.02.2021 09:20

Mathematics, 14.02.2021 09:20


Mathematics, 14.02.2021 09:20


Mathematics, 14.02.2021 09:20

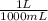 ) = .236 L
) = .236 L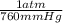 )= 0.97 atm
)= 0.97 atm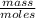 or MM=
or MM= 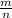 . They're all the same.
. They're all the same.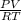 , where R (constant)= 0.082 L atm mol-1 K-1
, where R (constant)= 0.082 L atm mol-1 K-1