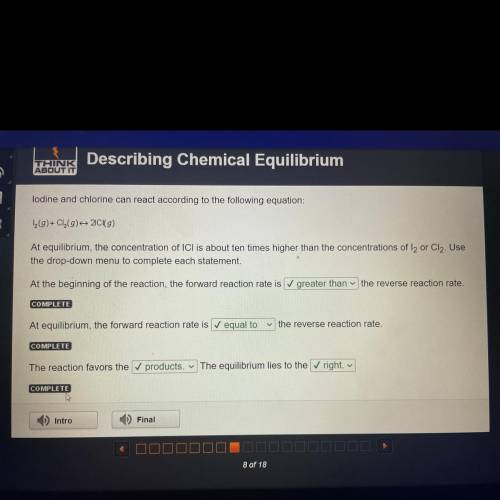
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At eq...

Chemistry, 26.04.2021 07:40 kinglightskin2k
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At equilibrium, the concentration of ICI is about ten times higher than the concentrations of l2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is greater than the reverse reaction rate.
COMPLETE
At equilibrium, the forward reaction rate is equal to
the reverse reaction rate.
COMPLETE
The reaction favors the ✓ products. The equilibrium lies to the right.
COMPLETE

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Questions

Mathematics, 04.12.2020 01:20

Health, 04.12.2020 01:20




English, 04.12.2020 01:20

Mathematics, 04.12.2020 01:20

Mathematics, 04.12.2020 01:20

History, 04.12.2020 01:20

Mathematics, 04.12.2020 01:20

Mathematics, 04.12.2020 01:20

English, 04.12.2020 01:20

History, 04.12.2020 01:20


Mathematics, 04.12.2020 01:20

Mathematics, 04.12.2020 01:20

Computers and Technology, 04.12.2020 01:20


Mathematics, 04.12.2020 01:20



