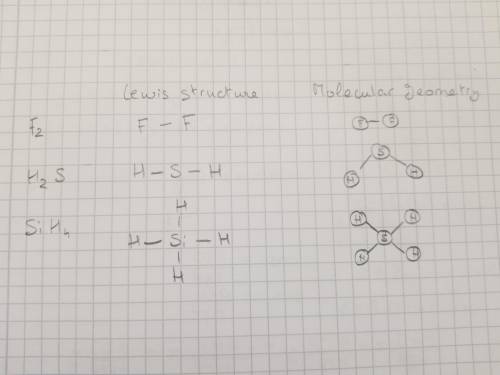
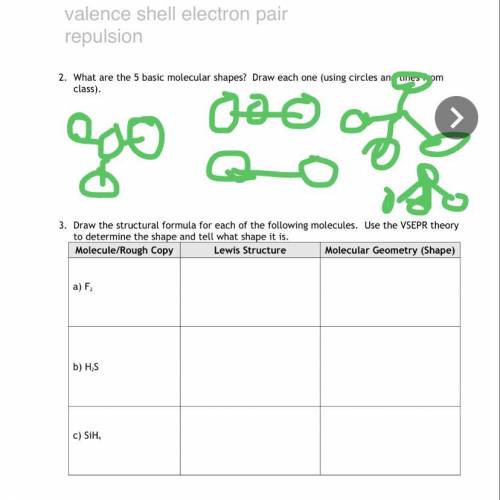
Can someone do the rest of this for me i missed school the day we did it
...

Chemistry, 25.04.2021 19:40 rclara34oxbrr9
Can someone do the rest of this for me i missed school the day we did it

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Questions

Mathematics, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10

English, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10

English, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10


Mathematics, 13.09.2021 16:10

Physics, 13.09.2021 16:10


Computers and Technology, 13.09.2021 16:10


Mathematics, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10

History, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10

Mathematics, 13.09.2021 16:10


Mathematics, 13.09.2021 16:10