
Chemistry, 24.04.2021 01:00 yesimabadbih5147
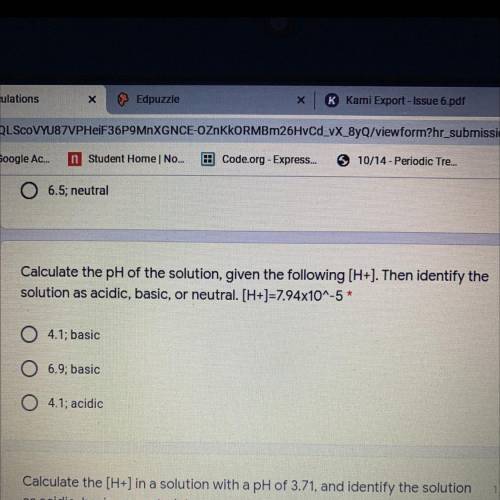
Calculate the pH of the solution, given the following (H+). Then identify the
solution as acidic, basic, or neutral. [H+]=7.94x10^-5 *
4.1; basic
0 6.9; basic
04.1; acidic

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

You know the right answer?
Calculate the pH of the solution, given the following (H+). Then identify the
solution as acidic,...
Questions

History, 29.07.2019 01:00




Health, 29.07.2019 01:00

English, 29.07.2019 01:00


History, 29.07.2019 01:00






History, 29.07.2019 01:00

Health, 29.07.2019 01:00

History, 29.07.2019 01:00



Computers and Technology, 29.07.2019 01:00



