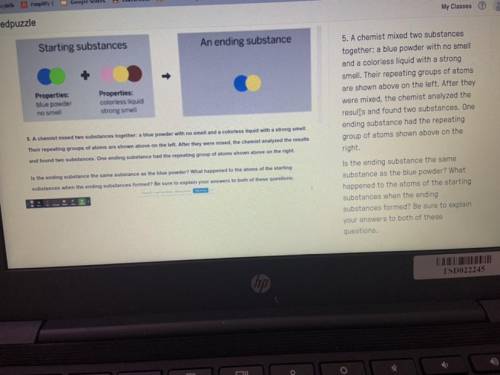
PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a col...

PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid with a strong
smell. Their repeating groups of atoms
are shown above on the left. After they
were mixed, the chemist analyzed the
results and found two substances. One
ending substance had the repeating
group of atoms shown above on the
right.
Is the ending substance the same
substance as the blue powder? What
happened to the atoms of the starting
substances when the ending
substances formed? Be sure to explain
your answers to both of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Questions




Mathematics, 21.04.2020 01:17

Mathematics, 21.04.2020 01:17

Biology, 21.04.2020 01:17




History, 21.04.2020 01:17

Geography, 21.04.2020 01:17

Mathematics, 21.04.2020 01:17


Mathematics, 21.04.2020 01:17








