
Chemistry, 23.04.2021 22:40 ibarral37102
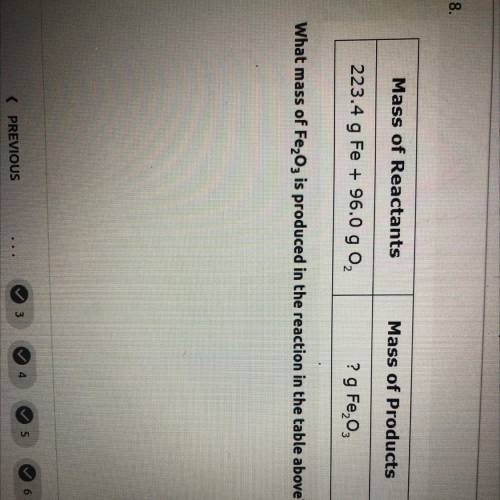
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0 g O2. and mass of products=? g Fe2O3

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0...
Questions

Mathematics, 28.01.2020 22:10

Health, 28.01.2020 22:10

World Languages, 28.01.2020 22:10

Mathematics, 28.01.2020 22:10


Mathematics, 28.01.2020 22:10


Health, 28.01.2020 22:10

Mathematics, 28.01.2020 22:10






Physics, 28.01.2020 22:10

Mathematics, 28.01.2020 22:10

Social Studies, 28.01.2020 22:10



Chemistry, 28.01.2020 22:10




