
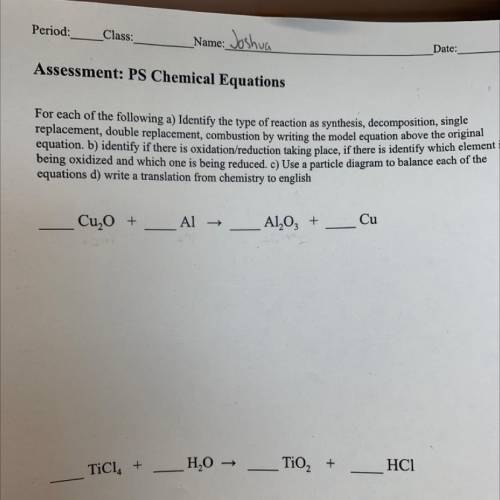
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as synthesis, decomposition, single
replacement, double replacement, combustion by writing the model equation above the original
equation, b) identify if there is oxidation/reduction
taking place, if there is identify which element i
being oxidized and which
one is being reduced. C) Use a particle diagram to balance each of the
equations d) write a translation from chemistry to english
Cu, O +
Al →
Al2O3 +
Cu
TiCl. +
H2O →
TiO2 +
HC1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as sy...
Questions

Social Studies, 04.08.2019 01:00

Business, 04.08.2019 01:00


Mathematics, 04.08.2019 01:00

Social Studies, 04.08.2019 01:00

Social Studies, 04.08.2019 01:00

Social Studies, 04.08.2019 01:00

Social Studies, 04.08.2019 01:00

Biology, 04.08.2019 01:00

Biology, 04.08.2019 01:00

Mathematics, 04.08.2019 01:00

Physics, 04.08.2019 01:00





Business, 04.08.2019 01:00

Computers and Technology, 04.08.2019 01:00




