
Chemistry, 23.04.2021 01:00 nicolecoulthard
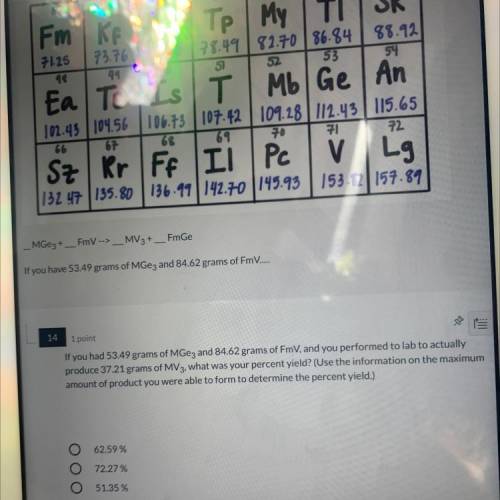
If you had 53.49 grams of MGez and 84.62 grams of FmV, and you performed to lab to actually produce 37.21 grams of MV3, what was your percent yield?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
If you had 53.49 grams of MGez and 84.62 grams of FmV, and you performed to lab to actually
produ...
Questions

Computers and Technology, 07.11.2020 05:00

Mathematics, 07.11.2020 05:00



Mathematics, 07.11.2020 05:00

Mathematics, 07.11.2020 05:00



English, 07.11.2020 05:00



Spanish, 07.11.2020 05:00







Computers and Technology, 07.11.2020 05:00

Mathematics, 07.11.2020 05:00



