
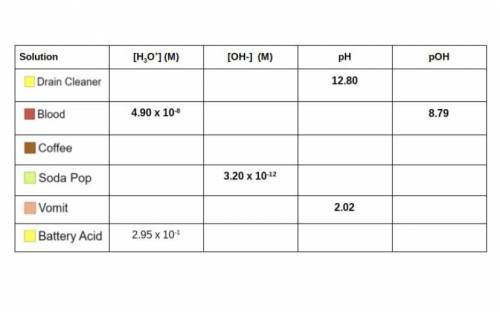
Use your knowledge of Molarity, pH/pOH, and the concentrations of ions to complete the table below. Some of
the work can be checked using the simulation. I have included a handy flow chart below the table with
common conversions when dealing with acids and bases. You will need to know how to do these calculations
for the unit test! (Picture attached)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Use your knowledge of Molarity, pH/pOH, and the concentrations of ions to complete the table below....
Questions

Social Studies, 06.10.2019 11:00

Spanish, 06.10.2019 11:00


Mathematics, 06.10.2019 11:00


Computers and Technology, 06.10.2019 11:00


History, 06.10.2019 11:00





Social Studies, 06.10.2019 11:00

Computers and Technology, 06.10.2019 11:00

Health, 06.10.2019 11:00


History, 06.10.2019 11:00





