
Chemistry, 22.04.2021 02:00 lexyjasmin6781
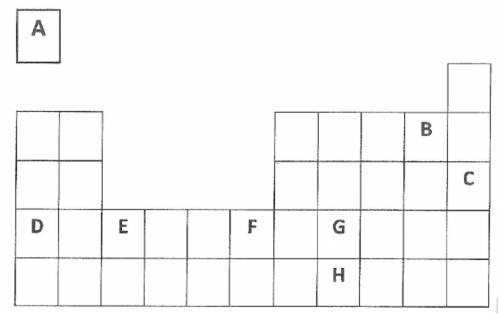
1. Which is the smallest/lightest element labeled above?
2. Name the three elements shown above that are nonmetals.
3. Which element is in the alkali metal family?
4. Which element is in the halogen family?
5. What number period are elements D, E, F, and G in?
6. What is the largest/heaviest element labeled above?
7. Which two elements would fall within the transition metals group?
8. Which element is in the noble gases family?
9. If element F had an atomic number of 22, what would be the atomic number of G?
a. What would be the atomic number of element E?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
1. Which is the smallest/lightest element labeled above?
2. Name the three elements shown above th...
Questions


Mathematics, 03.02.2021 23:30



Mathematics, 03.02.2021 23:30

Mathematics, 03.02.2021 23:30


Mathematics, 03.02.2021 23:30

Mathematics, 03.02.2021 23:30


Mathematics, 03.02.2021 23:30

Chemistry, 03.02.2021 23:30


Mathematics, 03.02.2021 23:30

Mathematics, 03.02.2021 23:30

History, 03.02.2021 23:30




Mathematics, 03.02.2021 23:30



