
Chemistry, 22.04.2021 01:00 mivantechenko9751
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacetate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the _ present in the buffer solution. The 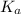 of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
b. Na⁺
c. chloroacetate ion
d. chloroacetic acid
e. This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacet...
Questions


Mathematics, 11.07.2019 10:00






History, 11.07.2019 10:00

English, 11.07.2019 10:00


Chemistry, 11.07.2019 10:00


Spanish, 11.07.2019 10:00

Spanish, 11.07.2019 10:00




Spanish, 11.07.2019 10:00

Mathematics, 11.07.2019 10:00



