
Chemistry, 21.04.2021 23:00 Trumpman137
The rusting of iron is represented by the balanced chemical equation below. If you have a 3.50 gram sample of iron, how many moles of Fe2O3 will there be after the iron has rusted (reacted) completely? PRO TIP: Don't enter units. Just enter the correct number in decimal format rounded to the correct number of significant figures.
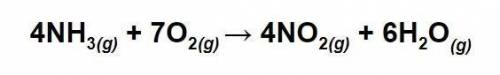

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
The rusting of iron is represented by the balanced chemical equation below. If you have a 3.50 gram...
Questions

World Languages, 05.05.2021 17:00


Mathematics, 05.05.2021 17:00



Mathematics, 05.05.2021 17:00

Mathematics, 05.05.2021 17:00

Mathematics, 05.05.2021 17:00

Mathematics, 05.05.2021 17:00


History, 05.05.2021 17:00





Mathematics, 05.05.2021 17:00


Mathematics, 05.05.2021 17:00

History, 05.05.2021 17:00



