
Chemistry, 21.04.2021 23:00 chanahvanya
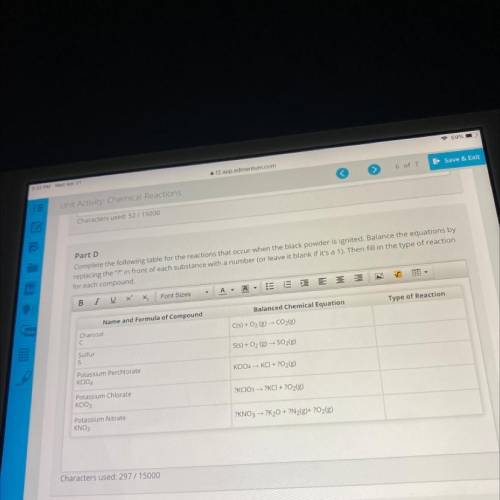
Part D
Complete the following table for the reactions that occur when the black powder is ignited. Balance the equations by
replacing the "?" in front of each substance with a number (or leave it blank if it's a 1). Then fill in the type of reaction
for each compound.
B.
I X
X
Font Sizes
A-
A-
= = 三 三 三
A
Name and Formula of Compound
Balanced Chemical Equation
Type of Reaction
Charcoal
C
C(s) + O2 (g) - CO2(g)
Sulfur
S
S(s) + O2(g) → SO2(g)
Potassium Perchlorate
KCIO4
KCIO4 - KCI + O2(g)
Potassium Chlorate
KCIO3
?KCIO3 → ?KCI + 2O2(g)
Potassium Nitrate
KNO3
?KNO3 → ?K20 + ?N2(g)+ ?O2(g)
Characters used: 297/ 15000

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Part D
Complete the following table for the reactions that occur when the black powder is ignited....
Questions




Mathematics, 10.12.2020 01:00


Spanish, 10.12.2020 01:00


History, 10.12.2020 01:00




Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Engineering, 10.12.2020 01:00



History, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00



