
Chemistry, 21.04.2021 21:20 RicoCheT89
calculate the amount of heat energy required to heat up 23.2 grams of ice from -21° C to 56° C ** please show your work**
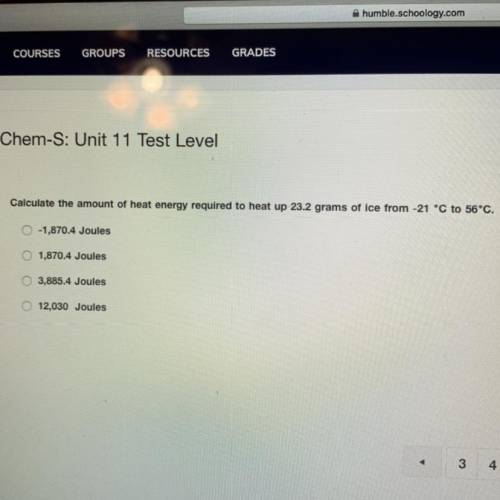

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
calculate the amount of heat energy required to heat up 23.2 grams of ice from -21° C to 56° C ** pl...
Questions


Mathematics, 19.05.2020 16:21

History, 19.05.2020 16:21

Chemistry, 19.05.2020 16:21


Mathematics, 19.05.2020 16:21








English, 19.05.2020 16:21

Social Studies, 19.05.2020 16:21



Mathematics, 19.05.2020 16:21


Biology, 19.05.2020 16:21



