Calculate the energy released from the reaction when 8.5g of O2 are reacted
...

Chemistry, 21.04.2021 16:50 capricorn0115
Calculate the energy released from the reaction when 8.5g of O2 are reacted
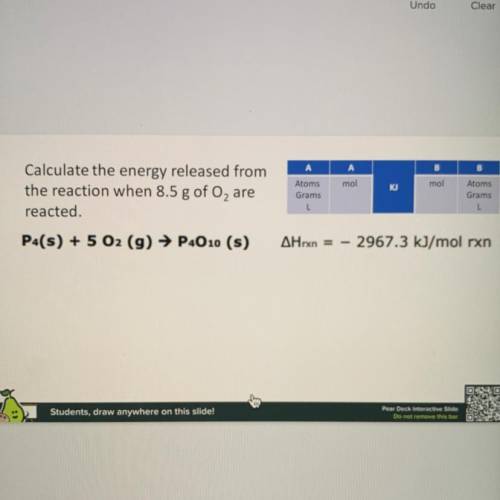

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions

Social Studies, 16.10.2020 05:01


Biology, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01



Mathematics, 16.10.2020 05:01


Chemistry, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


English, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Spanish, 16.10.2020 05:01




English, 16.10.2020 05:01



