Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
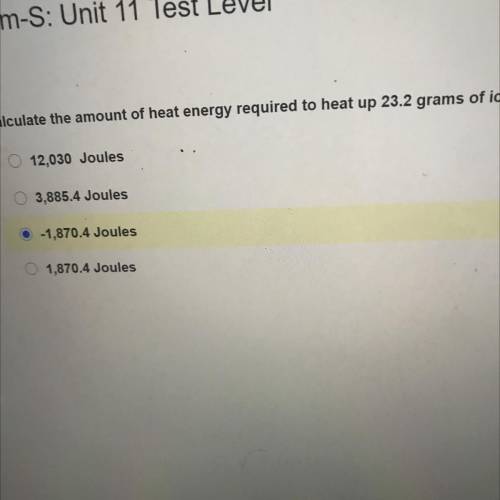
Chem-S: Unit 11 Test Level
Calculate the amount of heat energy required to heat up 23.2 grams of i...
Questions

Mathematics, 15.11.2020 22:10


Health, 15.11.2020 22:10

Mathematics, 15.11.2020 22:10

Mathematics, 15.11.2020 22:10



Mathematics, 15.11.2020 22:10

English, 15.11.2020 22:10

Medicine, 15.11.2020 22:10

Mathematics, 15.11.2020 22:10

English, 15.11.2020 22:10

Mathematics, 15.11.2020 22:10



English, 15.11.2020 22:10

Mathematics, 15.11.2020 22:10


Computers and Technology, 15.11.2020 22:10